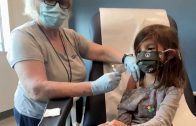
FDA panel backs Pfizer’s low-dose COVID-19 vaccine for kids
November 2, 2021 3:56 pm
The U.S. moved a step closer to expanding COVID-19 vaccinations for millions more children as government advisers on Tuesday endorsed kid-size doses of Pfizer’s shots for 5- to 11-year-olds.
Category: News